France In Vitro Diagnostics Market Trends, Size, Share, Demand, Revenue and Future Outlook
France In Vitro Diagnostics Market Growth, Size, Trends Analysis- By Test Type, By Technology, By Product Type, By Application, By End User- Regional Outlook, Competitive Strategies and Segment Forecast to 2033
| Published: Oct-2024 | Report ID: HLCA2482 | Pages: 1 - 105 | Formats*: |
| Category : Healthcare | |||
- April 2024; A number of French healthcare organizations have announced collaborations with AI-powered IVD firms to include machine learning for improved diagnostic precision. By providing quicker and more accurate findings, this is intended to improve patient outcomes, especially in the diagnosis of cancer and uncommon diseases.
- February 2023; Molecular diagnostics received a boost from the French government as part of a larger healthcare strategy. This action is to help the post-pandemic expansion of the IVD industry by enhancing the country's diagnostic capacity for infectious illnesses and customized medicine.

| Report Metric | Details |
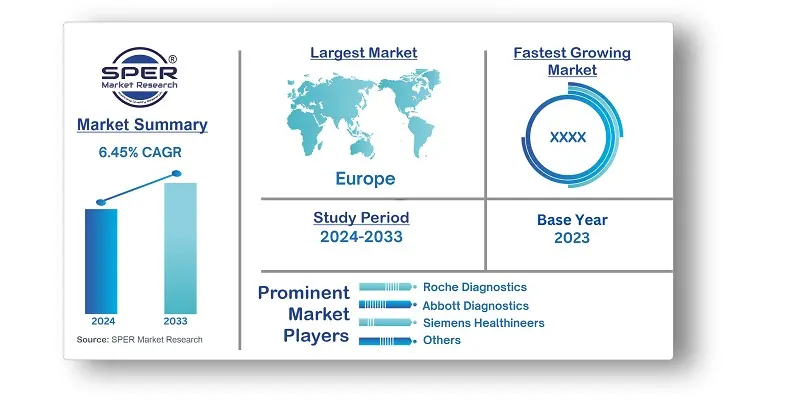
| Market size available for years | 2020-2033 |
| Base year considered | 2023 |
| Forecast period | 2024-2033 |
| Segments covered | By Test Type, By Technology, By Products Type, By Application, By End User. |
| Regions covered | Eastern, Western, Northern, Southern. |
| Companies Covered | Roche Diagnostics, Abbott Diagnostics, Siemens Healthineers, Danaher Corporation, Thermo Fisher Scientific, Sysmex Corporation, Qiagen N.V, Johnson & Johnson, Bayer AG, Bio-Rad Laboratories. |
- Healthcare Providers
- Diagnostic Laboratories
- Pharmaceutical Companies
- Medical Device Manufacturers
- Government and Regulatory Bodies
- Research Institutions and Universities
- Healthcare Professionals
- Patients and Patient Advocacy Groups
- Insurance Companies
- Investors and Venture Capitalists
| By Test Type: | |
| By Technology: | |
| By Product Type: | |
| By Application: | |
| By End User: |
- France In-Vitro Diagnostics Market Size (FY’2024-FY’2033)
- Overview of France In-Vitro Diagnostics Market
- Segmentation of France In-Vitro Diagnostics Market By Test Type (ELISA & CLIA, PCR, Rapid Test, Fluorescence Immunoassays, In Situ Hybridisation, Transcription Mediated Amplification, Colorimetric Immunoassay, Radioimmunoassay, Sequencing, Isothermal Nucleic Acid Amplification Technology, Others)
- Segmentation of France In-Vitro Diagnostics Market By Technology (Immunoassay, Clinical Chemistry, Molecular Diagnostics, Hematology, Microbiology, Coagulation, Others)
- Segmentation of France In-Vitro Diagnostics Market By Product Type (Services, Instruments, Reagent)
- Segmentation of France In-Vitro Diagnostics Market By Application (Infectious Disease, Diabetes, Cardiology, Oncology, Nephrology, Autoimmune Diseases, Drug Testing, Other Applications)
- Segmentation of France In-Vitro Diagnostics Market By End User (Hospitals, Laboratories, Home-Care, Others)
- Statistical Snap of France In-Vitro Diagnostics Market
- Expansion Analysis of France In-Vitro Diagnostics Market
- Problems and Obstacles in France In-Vitro Diagnostics Market
- Competitive Landscape in the France In-Vitro Diagnostics Market
- Impact of COVID-19 and Demonetization on France In-Vitro Diagnostics Market
- Details on Current Investment in France In-Vitro Diagnostics Market
- Competitive Analysis of France In-Vitro Diagnostics Market
- Prominent Players in the France In-Vitro Diagnostics Market
- SWOT Analysis of France In-Vitro Diagnostics Market
- France In-Vitro Diagnostics Market Future Outlook and Projections (FY’2024-FY’2033)
- Recommendations from Analyst
1.1. Scope of the report1.2. Market segment analysis
2.1. Research data source
2.1.1. Secondary Data2.1.2. Primary Data2.1.3. SPERs internal database2.1.4. Premium insight from KOLs
2.2. Market size estimation
2.2.1. Top-down and Bottom-up approach
2.3. Data triangulation
4.1. Driver, Restraint, Opportunity and Challenges analysis
4.1.1. Drivers4.1.2. Restraints4.1.3. Opportunities4.1.4. Challenges
4.2. COVID-19 Impacts of the France In Vitro Diagnostics Market
5.1. SWOT Analysis
5.1.1. Strengths5.1.2. Weaknesses5.1.3. Opportunities5.1.4. Threats
5.2. PESTEL Analysis
5.2.1. Political Landscape5.2.2. Economic Landscape5.2.3. Social Landscape5.2.4. Technological Landscape5.2.5. Environmental Landscape5.2.6. Legal Landscape
5.3. PORTERs Five Forces
5.3.1. Bargaining power of suppliers5.3.2. Bargaining power of buyers5.3.3. Threat of Substitute5.3.4. Threat of new entrant5.3.5. Competitive rivalry
5.4. Heat Map Analysis
6.1. France In Vitro Diagnostics Market Manufacturing Base Distribution, Sales Area, Product Type6.2. Mergers & Acquisitions, Partnerships, Product Launch, and Collaboration in France In Vitro Diagnostics Market
7.1. France In Vitro Diagnostics Market Size, Share and Forecast, By Test Type, 2020-20267.2. France In Vitro Diagnostics Market Size, Share and Forecast, By Test Type, 2027-20337.3. ELISA & CLIA7.4. PCR7.5. Rapid Test7.6. Fluorescence Immunoassays (FIA)7.7. In Situ Hybridization7.8. Transcription Mediated Amplification7.9. Colorimetric Immunoassay7.10. Radioimmunoassay (RIA)7.11. Sequencing7.12. Isothermal Nucleic Acid Amplification Technology7.13. Others
8.1. France In Vitro Diagnostics Market Size, Share and Forecast, By Technology, 2020-20268.2. France In Vitro Diagnostics Market Size, Share and Forecast, By Technology, 2027-20338.3. Immunoassay8.4. Clinical Chemistry8.5. Molecular Diagnostics/Genetics8.6. Hematology8.7. Microbiology8.8. Coagulation8.9. Others
9.1. France In Vitro Diagnostics Market Size, Share and Forecast, By Product Type, 2020-20269.2. France In Vitro Diagnostics Market Size, Share and Forecast, By Product Type, 2027-20339.3. Services9.4. Instruments9.5. Reagents
10.1. France In Vitro Diagnostics Market Size, Share and Forecast, By Application, 2020-202610.2. France In Vitro Diagnostics Market Size, Share and Forecast, By Application, 2027-203310.3. Infectious Disease10.4. Diabetes10.5. Cardiology10.6. Oncology10.7. Nephrology10.8. Autoimmune Diseases10.9. Drug Testing10.10. Other Applications
11.1. France In Vitro Diagnostics Market Size, Share and Forecast, By End User, 2020-202611.2. France In Vitro Diagnostics Market Size, Share and Forecast, By End User, 2027-203311.3. Hospitals11.4. Laboratories11.5. Home – Care11.6. Others
12.1. France In Vitro Diagnostics Market Size and Market Share By Region (2020-2026)12.2. France In Vitro Diagnostics Market Size and Market Share By Region (2027-2033)12.3. Eastern12.4. Western12.5. Northern12.6. Southern
13.1. Roche Diagnostics
13.1.1. Company details13.1.2. Financial outlook13.1.3. Product summary13.1.4. Recent developments
13.2. Abbott Diagnostics
13.2.1. Company details13.2.2. Financial outlook13.2.3. Product summary13.2.4. Recent developments
13.3. Siemens Healthineers
13.3.1. Company details13.3.2. Financial outlook13.3.3. Product summary13.3.4. Recent developments
13.4. Danaher Corporation
13.4.1. Company details13.4.2. Financial outlook13.4.3. Product summary13.4.4. Recent developments
13.5. Thermo Fisher Scientific
13.5.1. Company details13.5.2. Financial outlook13.5.3. Product summary13.5.4. Recent developments
13.6. Sysmex Corporation
13.6.1. Company details13.6.2. Financial outlook13.6.3. Product summary13.6.4. Recent developments
13.7. Qiagen N.V
13.7.1. Company details13.7.2. Financial outlook13.7.3. Product summary13.7.4. Recent developments
13.8. Johnson & Johnson
13.8.1. Company details13.8.2. Financial outlook13.8.3. Product summary13.8.4. Recent developments
13.9. Bayer AG
13.9.1. Company details13.9.2. Financial outlook13.9.3. Product summary13.9.4. Recent developments
13.10. Bio-Rad Laboratories
13.10.1. Company details13.10.2. Financial outlook13.10.3. Product summary13.10.4. Recent developments
13.11. Others
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.