Pharmacovigilance & Regulatory Affairs
Our Innovative PV Solutions ensures drug safety and regulatory compliance with End-to-end case management solutions, Risk and Signal management, Scientific writing services and Europe based QPPV services. We help companies to be future ready with transformative Consulting solutions and tool offerings.
Key services and functions
Key services and functions within pharmacovigilance (PV) and clinical research, organized into five main categories

Case Management
Focuses on adverse event handling (ICSR, SAE/SUSAR management), literature reviews, PV data migration, medical reviews, and PV agreements.

Scientific Writing Services
Supports clinical research documentation, including Clinical Study Reports (CSR), protocols, regulatory submissions (Common Technical Document), and patient-facing materials (Informed Consent forms).

Risk & Signal Management
Includes signal detection, benefit-risk assessments, US-REMS (Risk Evaluation and Mitigation Strategies), and responses to health inquiries.

Europe/UK Based Services
Tailored regulatory compliance: PV audits, QPPV (Qualified Person for Pharmacovigilance) services, and EU-specific systems (Eudravigilance, PEMF) setup/maintenance.

Consulting & Strategy
Strategic PV process optimization, regulatory intelligence, automation, and product/database solutions.
Our Coverage
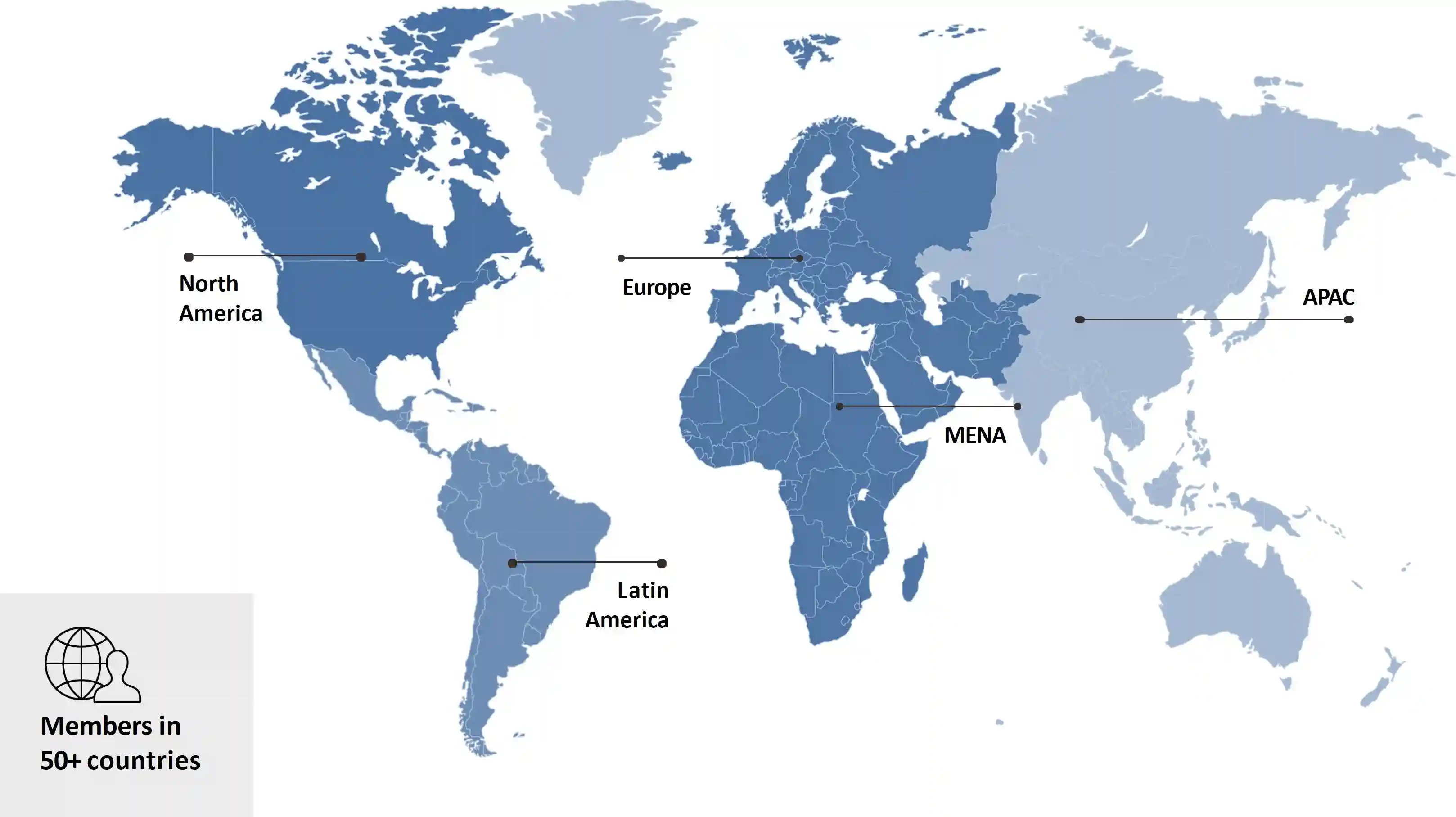
Global Access Through
Panel
+ Tailored Recruitment
Healthcare Panel

HCP's, Patients, Caregivers
Consumer Panel
members associated across globe
B2B Panel

Subject Matter Experts, Decision Makers, and Industry Leaders
Countries
Languages
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.