Vascular Closure Device Market Sales, Size, Demand and Key Manufacturers
Global Vascular Closure Device Market Size, Share & Trends Analysis Report By Type, By Access, By Procedure, Regional Outlook, Competitive Strategies and Segment Forecast - 2030
| Published: Jan-2022 | Report ID: MEDE2202 | Pages: 1 - 206 | Formats*: |
| Category : Medical Devices | |||
| Report Metric | Details |
| Market size available for years | 2019-2030 |
| Base year considered | 2021 |
| Forecast period | 2022-2030 |
| Segments covered | By Type, By Access, By Procedure |
| Geographies covered | North America, Europe, APAC, Latin America, and the Middle East & Africa |
| Companies Covered | Abbott, Cardinal Health, Medtronic, TZ Medical, Merit Medical Systems, Inc., Rex Medical, Vygon Company, Vasorum Ltd., Terumo Corporation, Cardiva Medical, Inc., Boston Scientific Corporation, Scion BioMedical, Morris Innovative, Inc., Vivasure Medical Ltd., Teleflex Incorporated, Tricol Biomedical, Transluminal Technologies, Meril Life Sciences Pvt. Ltd., Medas USA, Advanced Vascular Dynamics, Marine Polymer Technologies, Inc. |
4.1. Introduction4.2. Market Dynamics
4.2.1. Drivers4.2.2. Restraints4.2.3. Opportunities4.2.4. Challenges
4.3. COVID-19 Impact of the Vascular Closure Device Market4.4. Market Trends
5.1. Passive Approximators
5.1.1. Cotton plugs5.1.2. Sealant or gel-based devices5.1.3. Compression assists devices
5.2. Active Approximators
5.2.1. Suture-based devices5.2.2. Clip-based devices
5.3. External Hemostatic Devices
6.1. Femoral Access6.2. Radial Access
7.1. Interventional Cardiology7.2. Interventional Radiology/Vascular Surgery
8.1. North America
8.1.1. US8.1.2. Canada
8.2. Europe
8.2.1. Germany8.2.2. UK8.2.3. France8.2.4. Italy8.2.5. Spain8.2.6. Rest of Europe
8.3. Asia-Pacific
8.3.1. China8.3.2. Japan8.3.3. India8.3.4. Rest of Asia-Pacific
8.4. Rest of the World
8.4.1. Latin America8.4.2. Middle East & Africa
9.1. Introduction9.2. Market Share Analysis, By Key Players (2021)9.3. Competitive Scenario
9.3.1. Product Launches9.3.2. Partnerships, Collaborations and Agreements9.3.3. Acquisitions9.3.4. Expansions9.3.5. Other Developments
10.1. Abbott, Cardinal Health10.2. Advanced Vascular Dynamics10.3. Boston Scientific Corporation10.4. Cardiva Medical, Inc.10.5. Marine Polymer Technologies, Inc.10.6. Medas USA10.7. Medtronic10.8. Meril Life Sciences Pvt. Ltd.10.9. Merit Medical Systems, Inc.10.10. Morris Innovative, Inc.10.11. Rex Medical10.12. Scion BioMedical10.13. Teleflex Incorporated10.14. Terumo Corporation10.15. Transluminal Technologies10.16. Tricol Biomedical10.17. TZ Medical10.18. Vasorum Ltd.10.19. Vivasure Medical Ltd.10.20. Vygon Company
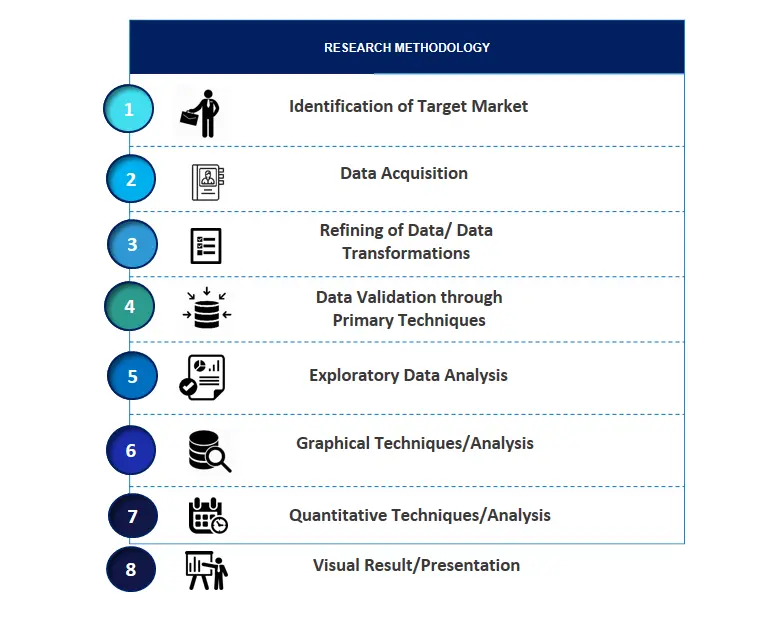
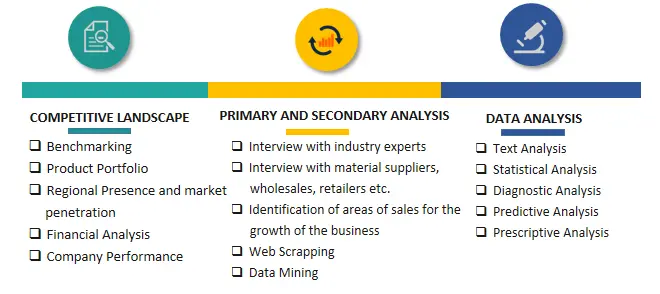
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.