Global High Potency APIs Market Insights, Size and Growth Forecast To 2030
High Potency APIs Market Size, Share & Trends Analysis By Type, By Synthesis, By Manufacturers, By Application, Regional Outlook, Competitive Strategies and Segment Forecasts to 2030
| Published: Jun-2022 | Report ID: PHAR2205 | Pages: 1 - 223 | Formats*: |
| Category : Pharmaceutical | |||
| Report Metric | Details |
| Market size available for years | 2019-2030 |
| Base year considered | 2021 |
| Forecast period | 2022-2030 |
| Segments covered | By Type, By Synthesis, By Manufacturers, By Application |
| Geographies covered | North America, Europe, Asia Pacific, Latin America, Middle East, Africa |
| Companies Covered | AbbVie, Albany Molecular Research Inc., BASF SE, Bayer AG, Boehringer Ingelheim International GmbH., Bristol-Myers Squibb Company, Carbogen Amcis, Cipla Inc., CordenPharma International, Dr. Reddy’s Laboratories Ltd., Eli Lilly and Company, F. Hoffmann-La Roche, Lonza, Merck & Co. Inc., Novartis International AG, Pfizer Inc., Sanofi, Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd., Viatris Inc. |
1.1. Scope of the report1.2. Market segment analysis
2.1 Research data source
2.1.1 Secondary data2.1.2 Primary data2.1.3 SPER’s internal database2.1.4 Premium insight from KOL’s
2.2 Market size estimation
2.2.1 Top-down and Bottom-up approach
2.3 Data triangulation
4.1. Driver, Restraint, Opportunity and Challenges analysis
4.1.1 Drivers4.1.2 Restraints4.1.3 Opportunities4.1.4 Challenges
4.2. COVID-19 Impacts of the High Potency APIs Market
5.1. SWOT analysis
5.1.1 Strengths5.1.2 Weaknesses5.1.3 Opportunities5.1.4 Threats
5.2. PESTEL analysis
5.2.1 Political landscape5.2.2 Economic landscape5.2.3 Social landscape5.2.4 Technological landscape5.2.5 Environmental landscape5.2.6 Legal landscape
5.3. PORTER’S five forces analysis
5.3.1 Bargaining power of suppliers5.3.2 Bargaining power of Buyers5.3.3 Threat of Substitute5.3.4 Threat of new entrant5.3.5 Competitive rivalry
5.4. Heat map analysis
6.1. Introduction6.2. Innovative High Potency APIs6.3. Generic High Potency APIs
7.1. Introduction7.2. Synthetic APIs
7.2.1. Synthetic APIs Market, By Type
7.3. Biotech APIs
7.3.1 Biotech APIs Market, By Type7.3.2 Biotech APIs Market, By Product7.3.3 Vaccines7.3.4 Recombinant Proteins
8.1. Introduction8.2. Captive High Potency APIs Manufacturers8.3. Merchant High Potency APIs Manufacturers
8.3.1 Merchant High Potency APIs Manufacturers Market, By Type8.3.2 Merchant High Potency APIs Manufacturers Market, By Type of Synthesis8.3.3 Synthetic APIs8.3.4 Biosynthetic APIs
9.1. Introduction9.2. Oncology9.3. Hormonal Imbalance9.4. Glaucoma9.5. Other Therapeutic Applications
10.1. North America
10.1.1. United States10.1.2. Canada10.1.3. Mexico
10.2. Europe
10.2.1. Germany10.2.2. United Kingdom10.2.3. France10.2.4. Italy10.2.5. Spain10.2.6. Rest of Europe
10.3. Asia-Pacific
10.3.1. China10.3.2. Japan10.3.3. India10.3.4. Australia10.3.5. South Korea10.3.6. Rest of Asia-Pacific
10.4. South America
10.4.1. Brazil10.4.2. Argentina10.4.3. Rest of South America
10.5. Middle East & Africa
10.5.1. Kingdom of Saudi Arabia10.5.2. United Arab Emirates10.5.3. Rest of Middle East & Africa
11.1. AbbVie
11.1.1. Company details11.1.2. Financial outlook11.1.3. Product summary11.1.4. Recent developments
11.2. Albany Molecular Research Inc.
11.2.1. Company details11.2.2. Financial outlook11.2.3. Product summary11.2.4. Recent developments
11.3. BASF SE
11.3.1. Company details11.3.2. Financial outlook11.3.3. Product summary11.3.4. Recent developments
11.4. Bayer AG
11.4.1. Company details11.4.2. Financial outlook11.4.3. Product summary11.4.4. Recent developments
11.5. Boehringer Ingelheim International GmbH.
11.5.1. Company details11.5.2. Financial outlook11.5.3. Product summary11.5.4. Recent developments
11.6. Bristol-Myers Squibb Company
11.6.1. Company details11.6.2. Financial outlook11.6.3. Product summary11.6.4. Recent developments
11.7. Carbogen Amcis
11.7.1. Company details11.7.2. Financial outlook11.7.3. Product summary11.7.4. Recent developments
11.8. Cipla Inc.
11.8.1. Company details11.8.2. Financial outlook11.8.3. Product summary11.8.4. Recent developments
11.9. Corden Pharma International
11.9.1. Company details11.9.2. Financial outlook11.9.3. Product summary11.9.4. Recent developments
11.10. Dr. Reddy’s Laboratories Ltd.
11.10.1. Company details11.10.2. Financial outlook11.10.3. Product summary11.10.4. Recent developments
11.11. Eli Lilly and Company
11.11.1. Company details11.11.2. Financial outlook11.11.3. Product summary11.11.4. Recent developments
11.12. F. Hoffmann-La Roche
11.12.1. Company details11.12.2. Financial outlook11.12.3. Product summary11.12.4. Recent developments
11.13. Lonza
11.13.1. Company details11.13.2. Financial outlook11.13.3. Product summary11.13.4. Recent developments
11.14. Merck & Co., Inc.
11.14.1. Company details11.14.2. Financial outlook11.14.3. Product summary11.14.4. Recent developments
11.15. Novartis International AG
11.15.1. Company details11.15.2. Financial outlook11.15.3. Product summary11.15.4. Recent developments
11.16. Pfizer, Inc.
11.16.1. Company details11.16.2. Financial outlook11.16.3. Product summary11.16.4. Recent developments
11.17. Sanofi
11.17.1 Company details11.17.2 Financial outlook11.17.3 Product summary11.17.4 Recent developments
11.18. Sun Pharmaceutical Industries, Ltd.
11.18.1. Company details11.18.2. Financial outlook11.18.3. Product summary11.18.4. Recent developments
11.19. Teva Pharmaceutical Industries Ltd.
11.19.1. Company details11.19.2. Financial outlook11.19.3. Product summary11.19.4. Recent developments
11.20. Viatris Inc.
11.20.1. Company details11.20.2. Financial outlook11.20.3. Product summary11.20.4. Recent developments
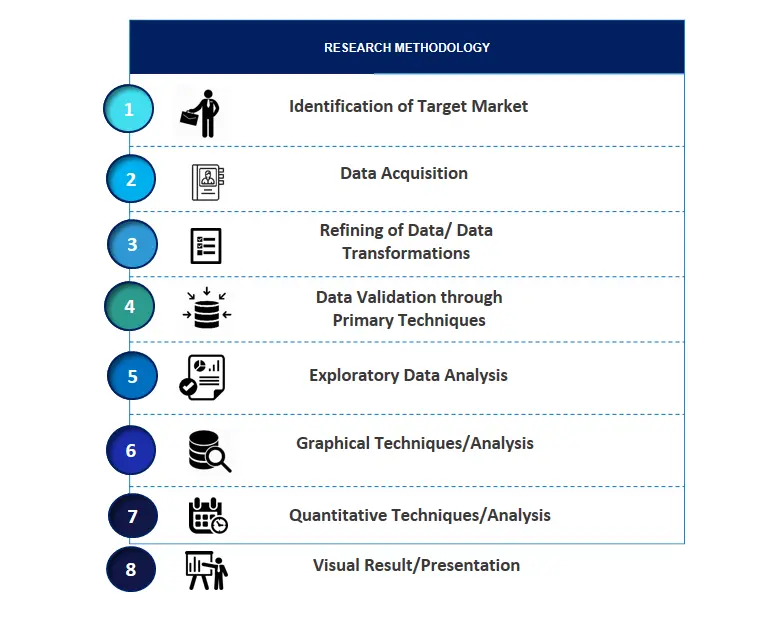
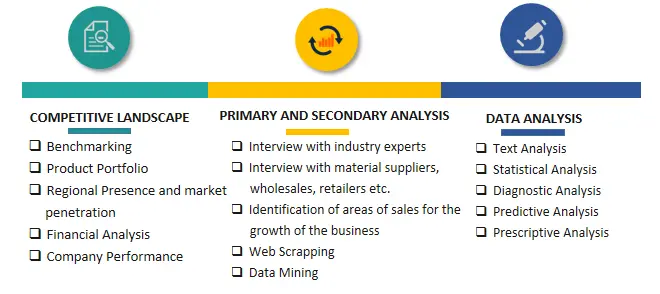
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.